Acid base
The theory
- Acids are substances which produce hydrogen ions in solution.
- Bases are substances which produce hydroxide ions in solution.
Limitations of the theory
Hydrochloric acid is neutralised by both sodium hydroxide solution and ammonia solution. In both cases, you get a colourless solution which you can crystallise to get a white salt - either sodium chloride or ammonium chloride.
These are clearly very similar reactions. The full equations are:
In the sodium hydroxide case, hydrogen ions from the acid are reacting with hydroxide ions from the sodium hydroxide - in line with the Arrhenius theory.
However, in the ammonia case, there don't appear to be any hydroxide ions!
But if you look at the equations carefully, the ammonia is in solution - NH3(aq). Ammonia reacts with water like this:
This is a reversible reaction, and in a typical dilute ammonia solution, about 99% of the ammonia remains as ammonia molecules. Nevertheless, there are hydroxide ions there and those react with hydrogen ions in just the same way as hydroxide ions from sodium hydroxide.
So you can just about justify ammonia as being a base on the Arrhenius definition - it does produce hydroxide ions in solution. But most of the reaction is going to be a direct reaction between ammonia molecules and hydrogen ions - which doesn't fit the Arrhenius definition.
This same reaction also happens between ammonia gas and hydrogen chloride gas.
In this case, there aren't any hydrogen ions or hydroxide ions in solution - because there isn't any solution. The Arrhenius theory wouldn't count this as an acid-base reaction, despite the fact that it is producing the same product as when the two substances were in solution. That's silly!
The Bronsted-Lowry Theory of acids and bases
The theory
- An acid is a proton (hydrogen ion) donor.
- A base is a proton (hydrogen ion) acceptor.
The Bronsted-Lowry theory doesn't go against the Arrhenius theory in any way - it just adds to it.
Hydroxide ions are still bases because they accept hydrogen ions from acids and form water.
An acid produces hydrogen ions in solution because it reacts with the water molecules by giving a proton to them.
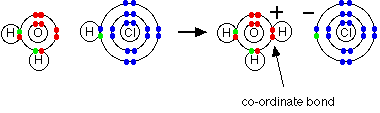
When hydrogen chloride gas dissolves in water to produce hydrochloric acid, the hydrogen chloride molecule gives a proton (a hydrogen ion) to a water molecule. A co-ordinate (dative covalent) bond is formed between one of the lone pairs on the oxygen and the hydrogen from the HCl. Hydroxonium ions, H3O+, are produced.
Lewis acids are electron pair acceptors. In the above example, the BF3 is acting as the Lewis acid by accepting the nitrogen's lone pair. On the Bronsted-Lowry theory, the BF3has nothing remotely acidic about it.
This is an extension of the term acid well beyond any common use.
What about more obviously acid-base reactions - like, for example, the reaction between ammonia and hydrogen chloride gas?
What exactly is accepting the lone pair of electrons on the nitrogen. Textbooks often write this as if the ammonia is donating its lone pair to a hydrogen ion - a simple proton with no electrons around it.
That is misleading! You don't usually get free hydrogen ions in chemical systems. They are so reactive that they are always attached to something else. There aren't any uncombined hydrogen ions in HCl.
There isn't an empty orbital anywhere on the HCl which can accept a pair of electrons. Why, then, is the HCl a Lewis acid?
Chlorine is more electronegative than hydrogen, and that means that the hydrogen chloride will be a polar molecule. The electrons in the hydrogen-chlorine bond will be attracted towards the chlorine end, leaving the hydrogen slightly positive and the chlorine slightly negative.https://bestnewyearmessage.blogspot.in/?m=1


Comments
Post a Comment
Please do not Enter any Spam Link In Comment Box